Language for Container Closure Integrity requirements in EU New Annex 1:(deadline for implementation 25 August 2023)
Global regulators are paying more and more attention to improving practices that assure good Container Closure Integrity of sterile products .
l USP<1207> Chapter ’Package integrity evaluation-sterile products’ implemented in August 2016 (old 2 pages chapter now 50+ pages)
l CDE ‘Technical Guide for research on container closure integrity for Chemical Injections’
l New EU Annex 1 ’Manufacture of sterile medicinal products’ revision-significant new language on container closure.
l Pharmaceutical inspection Co-operation scheme (PIC/S) and the world health Organization(WHO) participated in finalizing requirements.
EU New Annex 1: Sections 8.21-8.25 Container Closure Integrity(CCI)
Changes in the last several draft revisions
Huge implications for SVP and LVP bags
Appropriately validated physical integrity test methods.
Scientifically justified sampling plans.
Stable Inspection for Product life cycle
Container Integrity-----Sealing/Closing process
8.21 Final Containers should be closed by appropriately validated methods. (For manufactures need development)
Container Integrity testing -----Fusion sealed
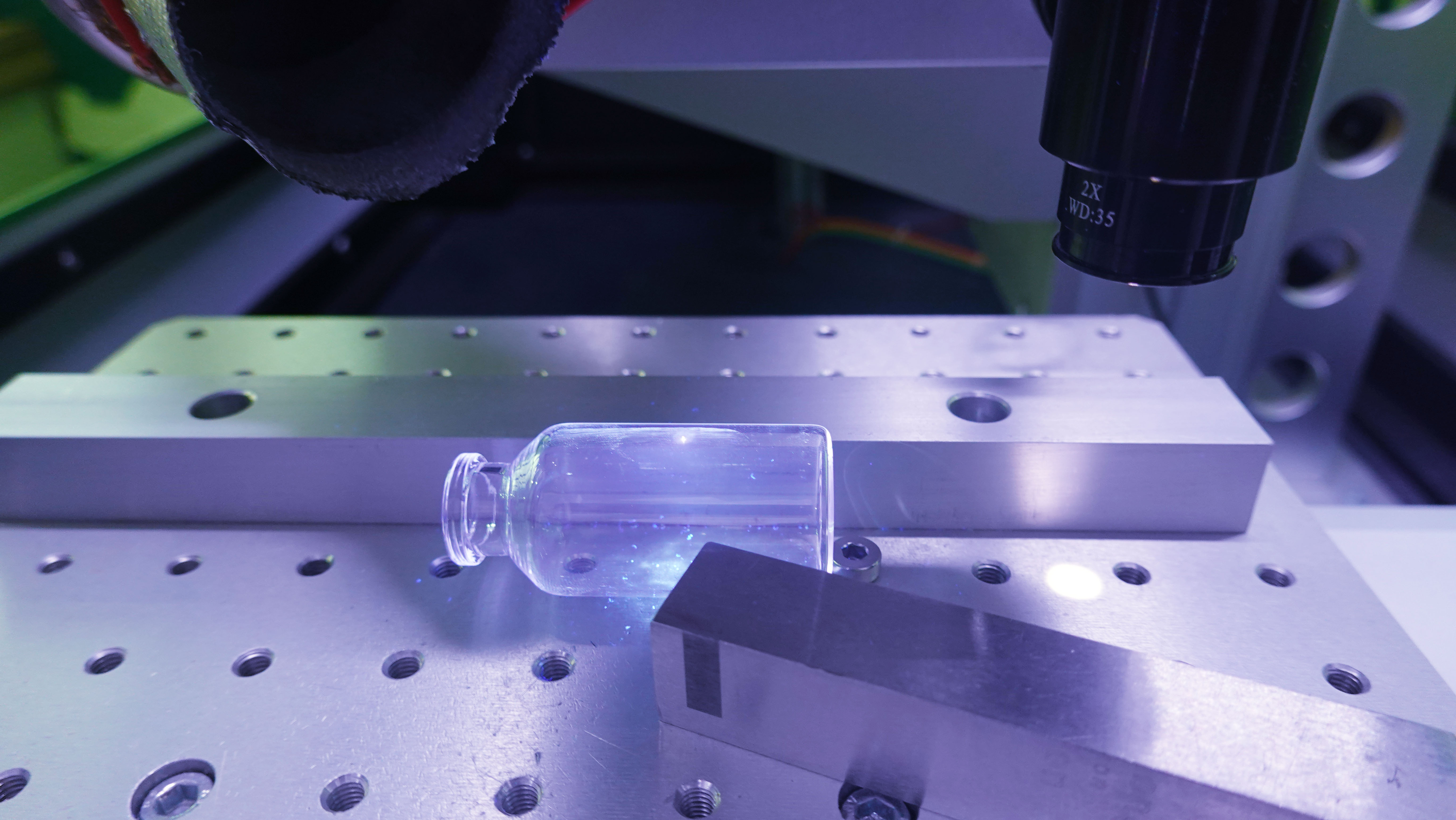
8.22 where final containers are closed by fusion, e.g. Blow-Fill-Seal(BFS), Form-Fill-Seal(FFS), Small and Large Volume Parenteral (SVL&LVP)bags, glass or plastic ampoules, the critical parameters and variables that affect seal integrity should be evaluated, determined, effectively controlled and monitored during operations. Glass ampoules, BFS units and small volume containers(≤100ml)closed by fusion should be subject to 100% integrity testing using validated methods. For large volume containers(>100ml)closed by fusion, reduced sampling maybe acceptable where scientifically justified and based on data demonstrating the consistency of the existing process, and a high level of process control. It should be noted that visual inspection is not considered as an acceptable integrity test method.

Container Integrity testing -----General
8.23 Samples of products using systems other than fusion should be taken and checked for integrity using validated methods. The frequency of testing should be based on the knowledge and experience of the container and closure systems being used. A scientifically justified sampling plan should be used. The sample size should be based on information such as supplier management, packaging component specifications and process knowledge.


Container Integrity-----Sealing/Vacuum retention
8.24 Container sealed under vacuum should be tested for maintenance of vacuum after an appropriate pre-determined period prior to certification/release and during shelf life.

Container Integrity-----transportation/shipping
8.25 The container closure integrity validation should take into consideration any transportation or shipping requirements that may negatively impact the integrity of the container(e.g. by decompression or extreme temperatures).
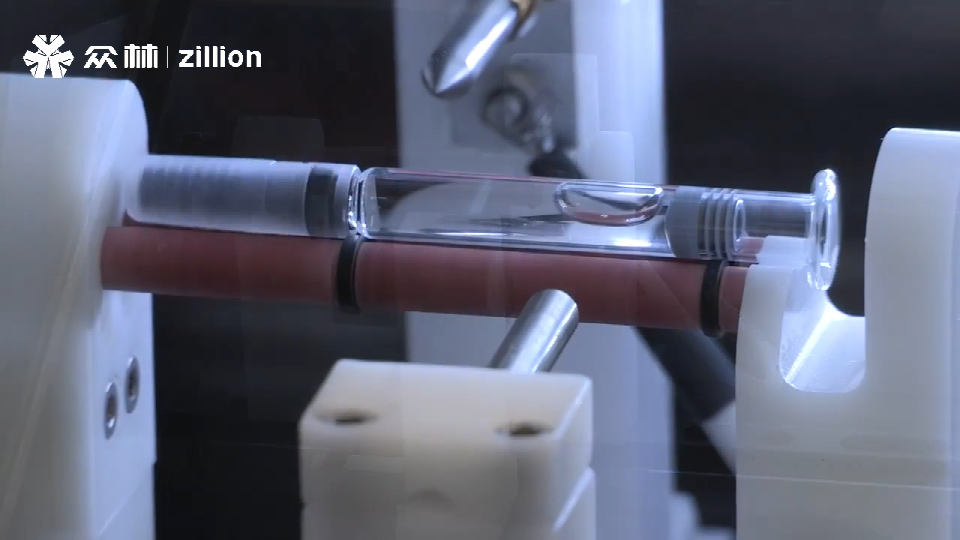
Examples:Syringe plunger movement during air freight; Ultra cold chain shipping.

Remarks: Improving Data Generation& Assurance of CCI
Following principles in USP<1207> is a approach for becoming compliant to CCI requirements in the new EU Annex 1.
USP<1207> Recommend Deterministic methods:
8.32 Where automated methods of inspection are used, the process should be validated to detect known defects (which may impact product quality or safety) and be equal to, or better than, manual inspection methods. The performance of the equipment should be challenged using representative defects prior to start up and at regular intervals throughout the batch.
8.33 Results of the inspection should be recorded and defect types and numbers trended. Reject levels for the various defect types should also be trended based on statistical principles. Impact to product on the market should be assessed as part of the investigation when adverse trends are observed.
Positive controls with calibrated leaks ensure that your leak testers are working properly, and are able to find even the most challenging leak artifacts.so that pharmaceutical companies can monitor and verify that their inspection systems meet proper quality standards.